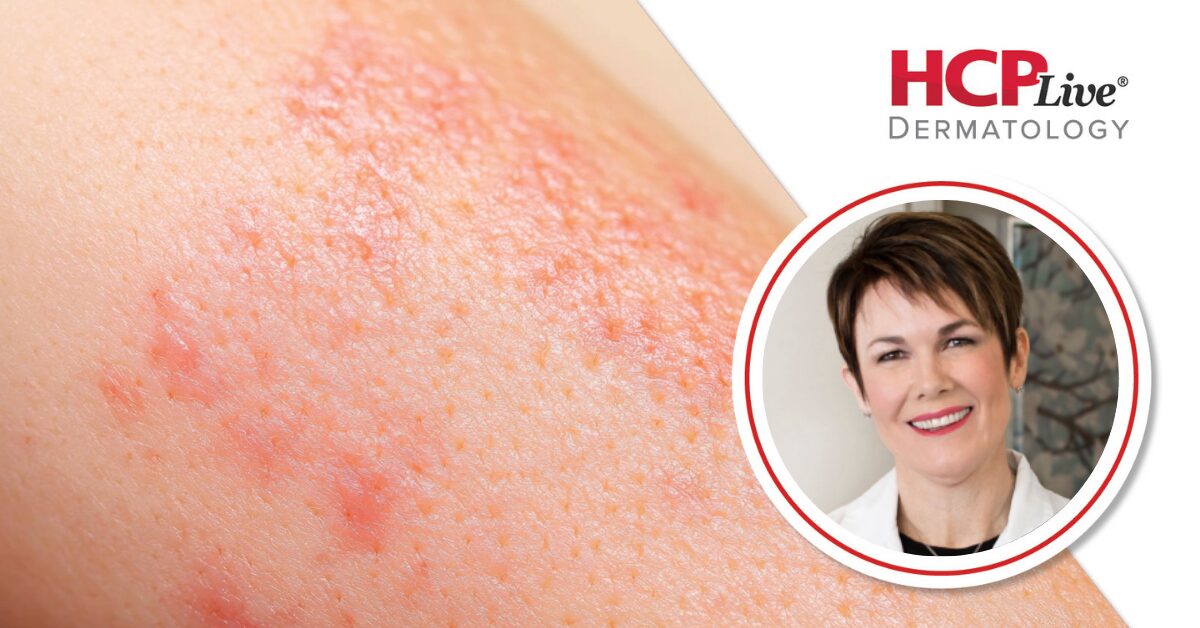
Takeda Pharmaceuticals announced on December 18, 2023, promising topline results from two pivotal Phase 3 studies of its drug, zasocitinib (TAK-279), aimed at treating adults with moderate-to-severe plaque psoriasis. The studies demonstrated that this next-generation, highly selective oral tyrosine kinase 2 (TYK2) inhibitor showed significant efficacy compared to placebo across primary endpoints, specifically the static Physician Global Assessment (sPGA) 0/1 and the Psoriasis Area and Severity Index (PASI) 75 scores.
To delve deeper into these findings, HCPLive spoke with Melinda Gooderham, MD, a dermatologist and medical director at the SKiN Centre for Dermatology in Ontario, Canada. Dr. Gooderham explained that the two studies, referred to as the Latitude studies, were designed as randomized, multicenter, double-blind trials that included both placebo and active comparator controls. Conducted across 21 countries, the trials involved the recruitment of 693 and 1,108 participants, respectively.
The co-primary endpoints of these studies assessed the proportion of patients achieving sPGA 0/1 and PASI 75 responses at the 16-week mark. According to Dr. Gooderham, the results indicated a significantly greater number of PASI 75 responses, observable as early as the 4-week mark and continuing to improve through Week 24. All 44 ranked secondary endpoints were met, including sPGA 0, PASI 90, and PASI 100, in comparisons with both placebo and apremilast.
Zasocitinib was generally well tolerated among participants, with safety findings aligning with previous research. Dr. Gooderham highlighted the potential of this once-daily oral agent to achieve complete skin clearance for individuals suffering from psoriasis. She remarked, “I think what’s exciting is that we’re seeing these levels of biologic efficacy. In the past, there has been a bit of a trade-off with our oral therapies being a little less effective than the biologics.”
Takeda’s announcement noted that the most common adverse events reported through the 24-week period included upper respiratory tract infections, acne, and nasopharyngitis. Importantly, no new safety signals were identified during the trials.
These findings are set to be presented at upcoming medical congresses, providing a platform for further discussion within the medical community. Additionally, Takeda plans to submit a New Drug Application to the U.S. Food and Drug Administration (FDA) and other regulatory authorities in 2026 to seek approval for zasocitinib.
Dr. Gooderham has reported nonfinancial support from Takeda, as well as personal fees from various pharmaceutical companies, including AbbVie, Amgen, and others. This context underscores the collaborative nature of research and development in the pharmaceutical industry, particularly in the pursuit of effective treatments for chronic conditions like psoriasis.
As the landscape of psoriasis treatment continues to evolve, the efficacy demonstrated by zasocitinib may represent a significant advancement for patients seeking effective oral therapies.