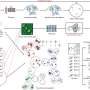
A recent study has provided significant insights into the operation of nuclear pore complexes (NPCs), the crucial gatekeepers that regulate the movement of materials between a cell’s nucleus and its cytoplasm. Researchers from The Rockefeller University, in collaboration with an international team led by the Hebrew University of Jerusalem, have produced a comprehensive map detailing the mechanisms of macromolecular transport through these intricate gateways. This research, published in PNAS, could catalyze advancements in medical and biotechnological fields.
The study reveals how NPCs, despite their minuscule size—about one five-hundredth the width of a human hair—manage to transport millions of molecules per minute while effectively filtering out others. The ability of these complexes to swiftly differentiate between various molecules has long puzzled scientists. According to Michael P. Rout, head of the Laboratory of Cellular and Structural Biology at The Rockefeller University, this research provides a clearer understanding of the dynamic processes at play within the NPCs.
Mapping the Molecular Landscape
For years, scientists have struggled to observe the inner workings of NPCs due to their tiny size. Previous models depicted NPCs as mechanical gates or static sieves, but these representations failed to account for the complexities observed in experimental settings. This new study integrates years of fragmented data into a cohesive computational framework, allowing researchers to simulate molecular interactions with unprecedented precision.
The researchers identified ten key molecular design features that contribute to the NPC’s efficiency and adaptability. A central aspect of their findings is the presence of a dense network of flexible protein chains known as FG repeats. These allow small molecules to navigate through the pore while restricting larger ones unless accompanied by nuclear transport receptors. The dynamic nature of this network facilitates rapid transport, akin to a “dance across a bridge,” as Rout describes, where only those with the right partners—transport receptors—are granted passage.
Lead author Barak Raveh from the Hebrew University noted that this study not only elucidates the transport mechanism but also predicts previously unobserved behaviors, enhancing our understanding of how NPCs operate under various conditions.
Implications for Health and Biotechnology
This research has far-reaching implications, particularly concerning diseases linked to malfunctions in the nuclear transport system, such as Alzheimer’s disease and ALS. Understanding how NPCs contribute to cellular processes may pave the way for developing targeted therapies aimed at these conditions, as indicated by Rout.
Moreover, the insights gained from this study could herald a new era in biotechnology. The model’s principles may be applied to engineer artificial nanopores that emulate NPC functions. Such innovations could revolutionize fields like targeted drug delivery and biosensing, making significant strides in medical technology.
Rout emphasizes that this research is only the beginning. The next steps involve diving deeper into the specific roles of different FG nucleoporins and cargo pathways. As the team continues to unravel the complexities of nuclear transport, the potential for comprehensive modeling of cellular systems grows, opening doors to unprecedented scientific exploration.
In conclusion, the collaboration between institutions such as The Rockefeller University and the Hebrew University of Jerusalem represents a significant leap forward in the understanding of cellular transport mechanisms, with promising applications in medicine and biotechnology on the horizon.