A new breakthrough in battery technology has emerged from the University of Adelaide, where researchers have developed a method to significantly reduce self-discharge in grid-scale aqueous zinc-iodine batteries. This advancement could position these batteries as viable alternatives to traditional lithium-ion systems, which dominate energy storage markets due to their high cost and safety concerns.
Rechargeable aqueous zinc batteries are increasingly recognized for their potential in large-scale energy storage. They offer advantages such as affordability, safety, and effective energy density. However, the conventional iodine cathodes commonly used in these batteries often suffer from slow reactions and inconsistent electrochemical performance.
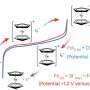
To address these challenges, a research team led by Professor Shizhang Qiao, Chair of Nanotechnology at the School of Chemical Engineering, explored the incorporation of ferrocene into the cathode chemistry. Their findings, published in the journal Nature Chemistry, reveal that ferrocene can enhance battery performance by effectively mitigating the polyiodides shuttle effect.
Enhancing Battery Performance with Ferrocene
The polyiodides shuttle effect occurs when intermediate compounds dissolve in the electrolyte and cycle between the battery’s cathode and anode, leading to energy loss. According to Professor Qiao, “The conversion of iodine in aqueous zinc-iodine batteries accompanies the polyiodides shuttle effect, but the conversion of ferrocene can precipitate the polyiodides, giving it a low self-discharge.”
Incorporating ferrocene not only improves energy density but also lowers the overall production cost of these batteries. Simulation results indicate that the new cathode chemistry can reduce the total battery cost by 9% compared to configurations lacking ferrocene. Additionally, the active mass in the cathode can reach an impressive 88%, minimizing capacity loss associated with inactive hosts.
A Scalable and Economical Solution
Professor Qiao highlighted the economic benefits of using ferrocene, noting that “since ferrocene is composed of low-cost elements, it offers favorable scalability and potentially low cost for large-scale production.” This makes ferrocene integration not only a practical solution for current battery technologies but also a strategy that could lead to more sustainable energy storage systems.
The research team’s findings represent a significant step forward in the quest for more efficient and cost-effective battery technologies. By addressing the limitations of traditional zinc-iodine batteries, this innovative approach could pave the way for broader adoption in various energy storage applications.
As the demand for reliable energy storage solutions continues to grow, advancements like this one from the University of Adelaide are poised to play a crucial role in transforming the energy landscape. The implications of this research extend beyond academic interest, potentially influencing the future of energy storage systems worldwide.