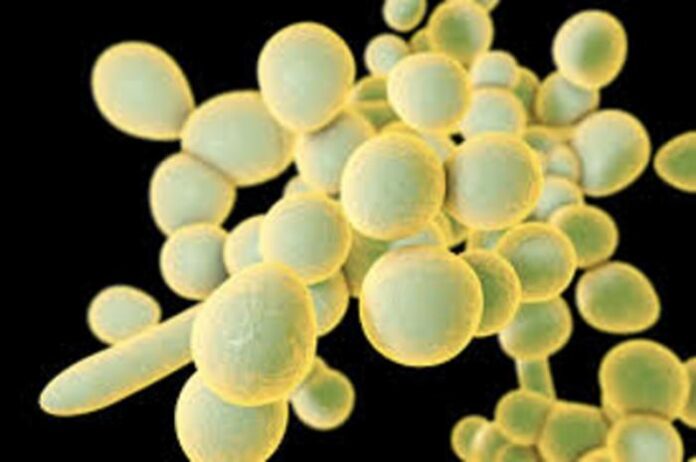
The development of a promising new fungal vaccine candidate, known as VXV-01, has received a significant boost with a contract from the National Institutes of Health (NIH) providing up to $40 million in funding. This initiative, a collaboration between the Lundquist Institute (TLI) and Vitalex Biosciences, aims to advance VXV-01 through to Phase I clinical trials, addressing the urgent need for effective treatments against invasive fungal diseases, particularly among hospitalized and immunocompromised patients.
Fungal infections caused by species such as Candida auris and Candida albicans are becoming increasingly problematic due to rising resistance to existing antifungal treatments. This situation has resulted in higher morbidity and mortality rates for affected individuals. The VXV-01 vaccine is designed as a dual-antigen candidate, targeting key opportunistic pathogens that pose significant risks in healthcare settings.
With the newly acquired funding, the team plans to commence manufacturing and preparations necessary for the clinical trials. Dr. Ashraf Ibrahim, the principal investigator and program lead for VXV-01 at TLI, emphasized the importance of this funding, stating, “Securing this contract is a pivotal moment for our team. By putting VXV-01 into development through human trials, we are significantly increasing our chances of making this vaccine a reality.”
Significance of the Research
The contract marks a major milestone for TLI’s vaccine research program, which has been at the forefront of life sciences in Los Angeles County since its founding in 1952. TLI is a nonprofit organization focused on addressing high-impact infectious disease challenges through innovative research. Vitalex, a start-up with an exclusive option to license VXV-01 for commercial development, aims to capitalize on the discoveries made in Dr. Ibrahim’s lab, which specializes in dual-antigen vaccines targeting multidrug-resistant infections.
In addition to VXV-01, the collaboration with Appili Therapeutics will enhance the development of a range of anti-infective therapies. Appili is recognized for advancing various treatments, including an FDA-approved metronidazole suspension and a vaccine candidate aimed at preventing tularemia, a serious biological threat.
As the project progresses, the potential impact of VXV-01 could extend beyond immediate health concerns, influencing broader public health strategies in combating fungal infections. The urgency of developing effective vaccines is underscored by the growing threat posed by antibiotic-resistant pathogens.
In conclusion, the federal funding awarded for the development of VXV-01 reflects a crucial step towards innovative solutions in the field of infectious diseases. With continued support and research, this vaccine candidate could significantly alter the landscape of treatment for vulnerable populations at risk from invasive fungal infections.