Research conducted by biologists at Dartmouth College has uncovered new insights into how embryos transition from rapid cell division to specialized cell development. This study, published in EMBO Reports in March 2024, focuses on the genetic mechanisms that guide this critical phase of embryonic growth, using Drosophila, commonly known as fruit flies, as a model organism.
After fertilization, embryos undergo a series of rapid cell divisions. During this process, they eventually slow down to develop specialized cells that perform distinct functions within the body. The signals that trigger this pivotal shift have remained largely elusive until now. Senior author Amanda Amodeo, an assistant professor of biological sciences at Dartmouth, explains, “The way that the DNA is packaged changes pretty dramatically in the very early embryo, as it goes from maternal control to transcribing its own genes.”
This research reveals that the transition is related to the embryo’s nuclear-to-cytoplasmic ratio. These findings could have significant implications for understanding certain cancers and aging in humans, conditions marked by disruptions in this ratio.
Fruit Flies as a Model for Developmental Biology
Fruit flies are frequently used in developmental biology studies due to the extensive genetic tools available for them and their transparent embryos, which allow for detailed imaging. The embryos, about the size of a needle tip, hatch within 24 hours, making them ideal for rapid experiments. Amodeo notes, “Drosophila has, time and time again, helped researchers identify fundamental biological processes that end up being important for human and animal well-being, and we hope that’s also the case here.”
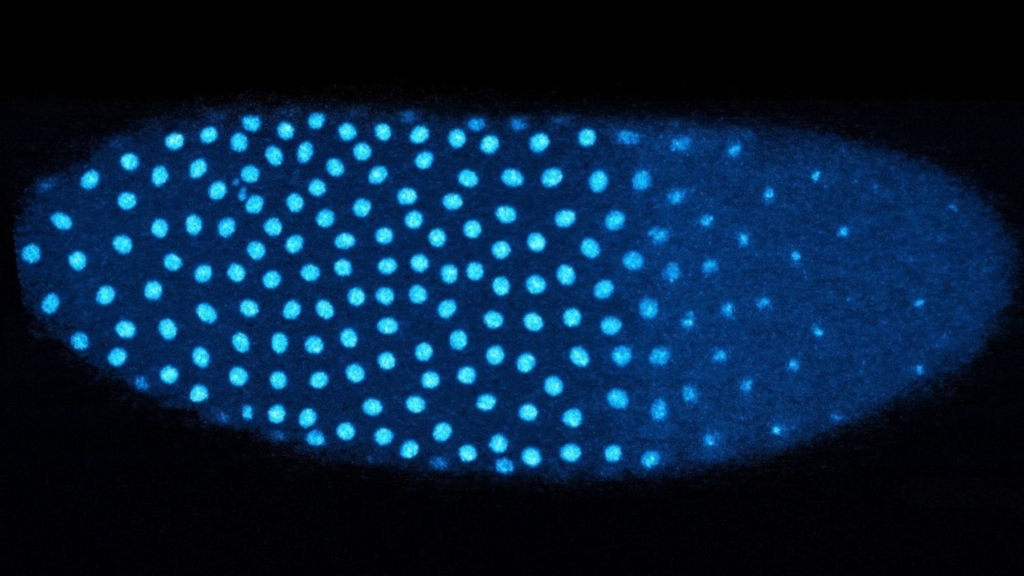
During early development, embryos rely on maternal proteins inherited from the mother. These proteins guide early processes before the embryo begins producing its own proteins. After approximately 13 rounds of synchronized cell division, the embryo transitions to controlling its development. At this stage, a single nucleus multiplies into over 6,000 nuclei.
Amodeo hypothesized that histone proteins might be key in coordinating the transition from maternal to embryonic control. Histones are essential for organizing and protecting DNA. Although previous research identified specific histones associated with gene activation in adult cells, their role in embryonic development remained largely unexamined.
Key Findings on Histone Functionality
The Dartmouth team discovered that the transition in early fruit fly embryos is associated with a histone called H3.3, which replaces histone H3 when the embryo senses a high concentration of nuclei. The researchers demonstrated that as cell division occurs, the amount of H3 associated with the embryo’s DNA decreases, while H3.3 levels increase. This switch is triggered when embryos detect they have become crowded with nuclei.
In embryos lacking the ability to distribute their nuclei evenly, areas with higher nuclei concentrations exhibited increased levels of H3.3, while less crowded regions did not undergo the same shift. This suggests that the distribution of nuclei plays a crucial role in the timing of the transition from maternal to embryonic control.
First author, Anusha Bhatt, a Guarini PhD student in Amodeo’s lab, emphasized the functional differences between histones, stating, “Even though they only differ by four amino acids, H3 and H3.3 have very different functions.” She elaborated that H3 is primarily involved in copying the genome, while H3.3 is utilized in specific genomic regions where gene transcription is necessary.
Going forward, the research team plans to explore the role of H3.3 in genome activation further. Bhatt expressed her interest in pinpointing where H3.3 is incorporated in the genome during the initial cell cycles and what specific genes it activates.
The study also includes contributions from two undergraduate students at Dartmouth, Madeleine Brown, who is now a PhD student at Cornell University, and Aurora Wackford, currently enrolled at Dartmouth. Amodeo expressed pride in their involvement, stating, “Anusha is the first graduate student to join my lab at Dartmouth, and Maddie was one of the first undergraduates to join my lab, so I’m very proud of both of them and this work.”
This research not only enhances the understanding of embryonic development in fruit flies but also sets the stage for future investigations into similar mechanisms in humans, potentially leading to advances in medical science and treatments for developmental disorders.