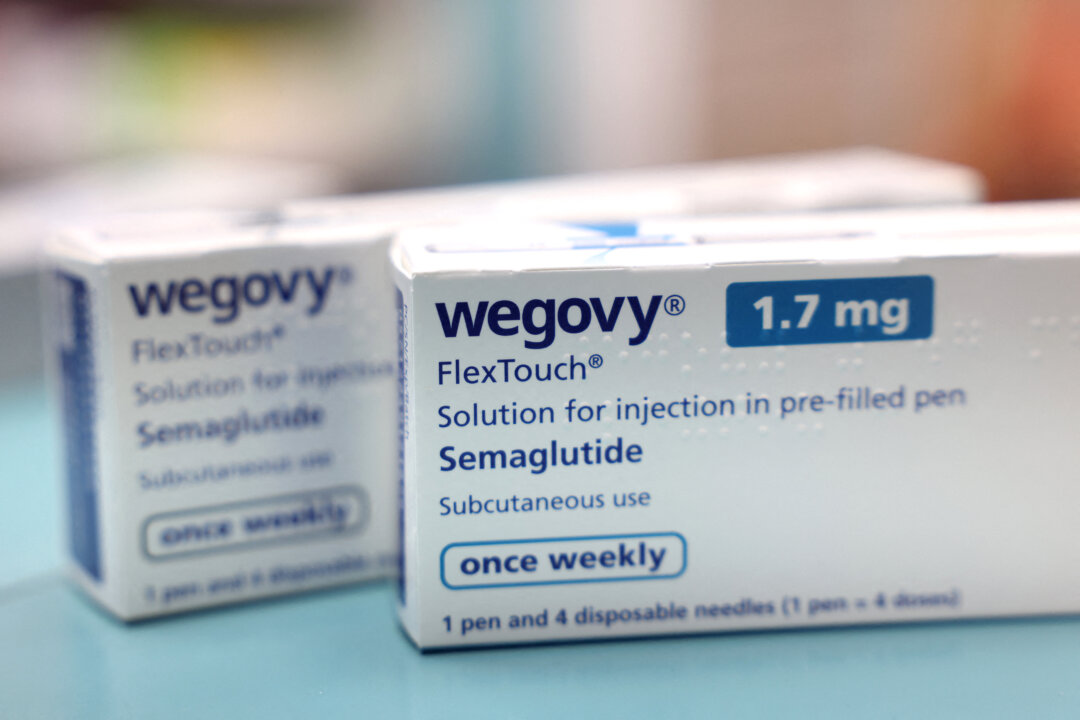
Multiple lots of the weight-loss medication Wegovy have been recalled by the pharmaceutical company Novo Nordisk after hair was discovered in prefilled syringes. The recall affects specific lots of Wegovy, which contains the active ingredient semaglutide. The notices were published by the Food and Drug Administration (FDA) on December 31, 2023, detailing the potential contamination issue.
The recall includes four lots of Wegovy single-dose prefilled pens in the 0.5 mg/0.5 mL formulation. Additionally, four lots of the 1 mg/0.5 mL version of the same product are also being recalled. According to the FDA notices, the recall was initiated due to the “presence of particulate matter,” specifically identifying that hair was found in a prefilled syringe.
Consumers who have purchased Wegovy from the affected lots are advised to stop using the product immediately. The FDA recommends that individuals consult their healthcare providers regarding any concerns and to return the medication to the pharmacy or dispose of it safely.
In light of this incident, Novo Nordisk has stated that it is conducting a thorough investigation to determine how the contamination occurred. The company is committed to maintaining high safety and quality standards for its products and is working closely with regulatory authorities to address the issue.
This recall raises concerns for many patients relying on Wegovy for weight management. The medication, which is administered via injection, has gained popularity for its effectiveness in helping individuals manage their weight by regulating appetite and food intake.
As the investigation continues, the FDA will monitor the situation and provide updates as necessary. Patients are encouraged to stay informed through official channels to ensure their safety and well-being.