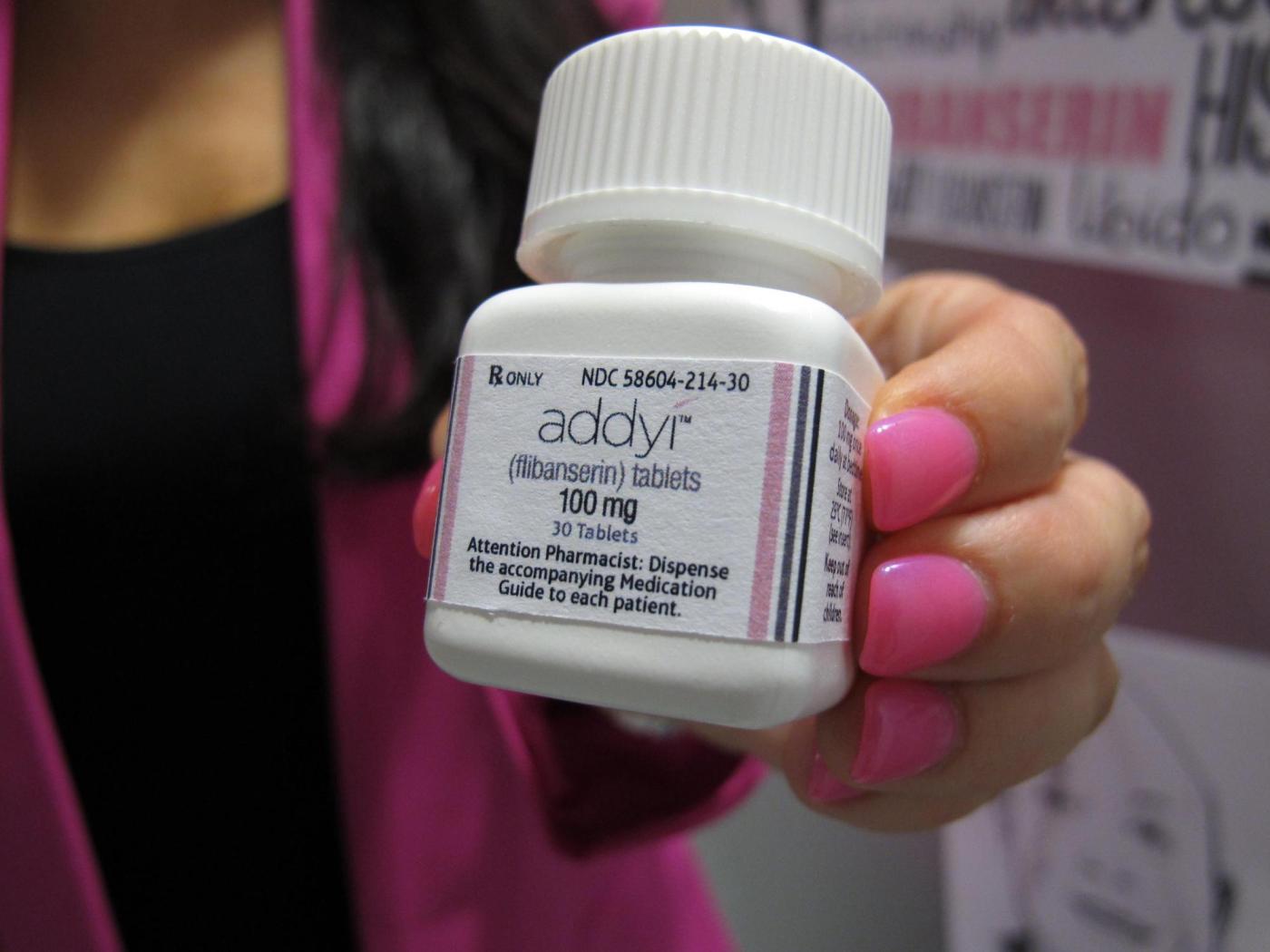
U.S. health officials have expanded the approval of the libido-boosting pill, Addyi, allowing women over the age of 65 who have gone through menopause to use the medication. The announcement from the Food and Drug Administration (FDA) was made on March 4, 2024, marking a significant shift in the drug’s accessibility for older women experiencing low sexual desire.
Addyi, initially approved in 2015, was designed primarily for premenopausal women facing emotional distress due to low libido. The pill, developed by Sprout Pharmaceuticals, is taken once a day and affects brain chemicals linked to mood and appetite. The FDA’s recent approval reflects ongoing efforts to address women’s sexual health and the complexities surrounding it.
The drug, however, carries a boxed warning—the most serious type issued by the FDA—regarding potential risks when combined with alcohol. These risks include dangerously low blood pressure and fainting, alongside side effects such as dizziness and nausea. Despite its initial promise, sales of Addyi have been limited, partly due to these concerns.
Cindy Eckert, CEO of Sprout Pharmaceuticals, expressed her satisfaction with the FDA’s decision, stating, “This approval reflects a decade of persistent work with the FDA to fundamentally change how women’s sexual health is understood and prioritized.” The company, headquartered in Raleigh, North Carolina, indicated that this update opens new avenues for addressing hypoactive sexual desire disorder, a condition recognized since the 1990s that affects many American women.
Diagnosing hypoactive sexual desire disorder can be challenging. Various factors—including hormonal changes, relationship issues, medical conditions, and mental health—can influence libido, particularly for women after menopause. Health professionals are tasked with ruling out these potential influences before prescribing medications like Addyi. Some psychologists even argue against classifying low sex drive as a medical issue, further complicating the diagnosis.
The initial approval of Addyi was not straightforward. The FDA rejected the drug twice due to its modest effectiveness and concerning side effects before eventually granting approval following a lobbying campaign by Sprout and advocacy group Even the Score, which framed the lack of treatment options for women as a critical women’s rights issue.
In 2019, the FDA approved a second treatment for low female libido, a different on-demand injection that targets various neurological mechanisms. The market for female sexual health products continues to evolve, but Addyi remains a focal point due to its controversial history and the ongoing conversation around women’s health needs.
As the dialogue surrounding female libido and treatment options progresses, the FDA’s recent decision signifies a growing acknowledgment of the importance of addressing sexual health issues that affect women across different life stages. The implications of this approval may lead to increased awareness and treatment options for women experiencing low sexual desire, particularly in older demographics.