A trustee of the American Medical Association (AMA) has publicly criticized the Centers for Disease Control and Prevention’s (CDC) recent decision to modify recommendations regarding the hepatitis B vaccine. The vote by the Advisory Committee on Immunization Practices (ACIP) to alter the guidance on the birth-dose recommendation has been labeled as “reckless” by Dr. Gerald Harmon, who has urged the CDC to reconsider its stance.
Dr. Harmon, who serves as the president of the AMA, expressed his concerns following the ACIP’s decision on March 15, 2024. The panel’s recommendation proposed a significant change, suggesting that the birth-dose of the hepatitis B vaccine could be optional for certain infants. This shift has sparked a heated debate within the medical community, as hepatitis B is a serious viral infection that can lead to chronic disease and liver failure.
“The ACIP’s vote to weaken the birth-dose recommendation for the hepatitis B vaccine is reckless and undermines the public health efforts that have been put in place to reduce the incidence of this potentially fatal disease,” Dr. Harmon stated. He emphasized the importance of maintaining robust vaccination protocols to protect vulnerable populations, particularly infants who are at high risk for hepatitis B infection.
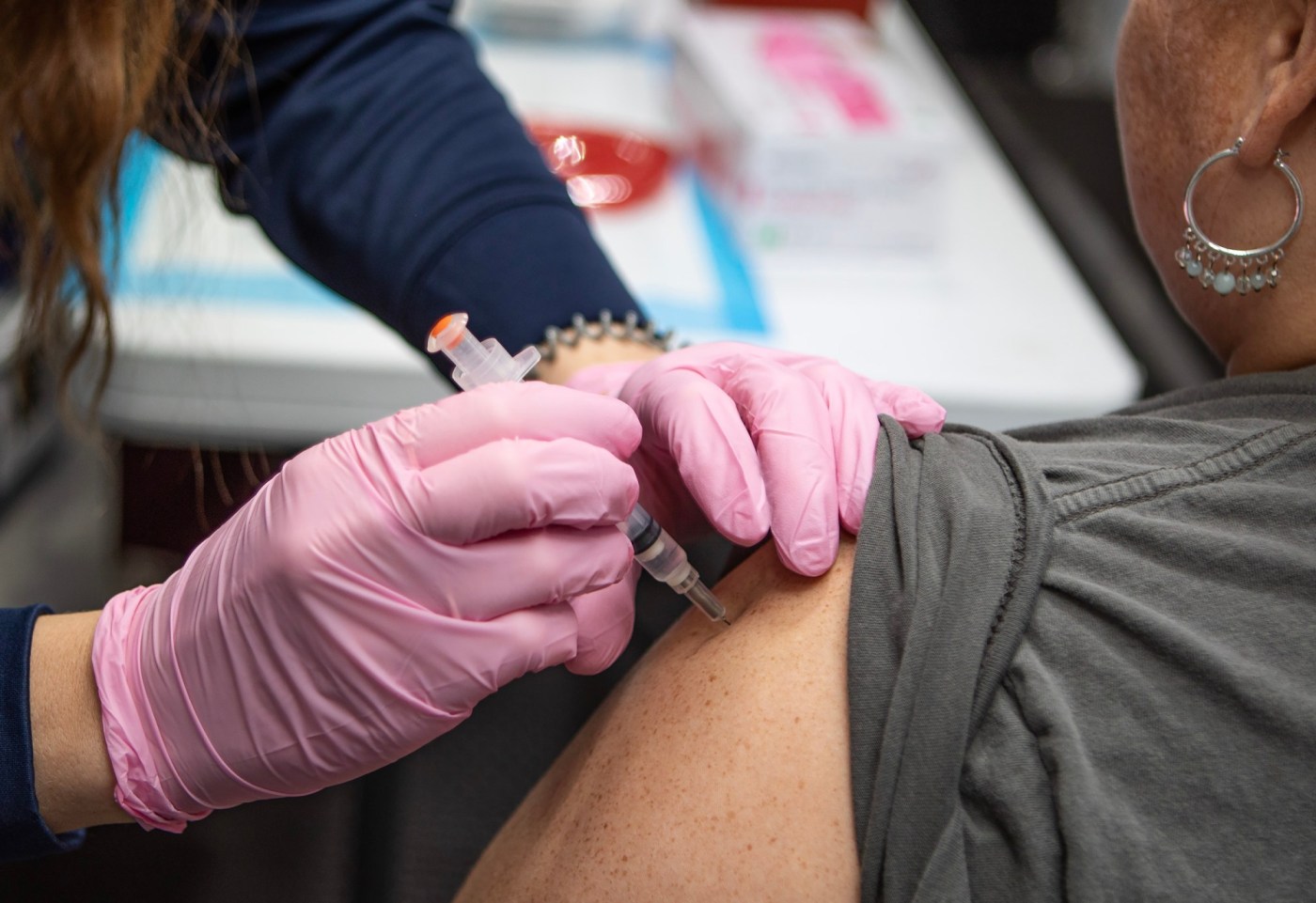
The CDC’s guidelines historically recommend that all newborns receive the hepatitis B vaccine within 24 hours of birth. This early vaccination is crucial in preventing the transmission of the virus from mother to child during delivery. According to the CDC, the hepatitis B vaccine is safe and effective, and it has played a significant role in decreasing the number of new infections in the United States.
Dr. Harmon highlighted that the potential consequences of loosening these recommendations could be dire. “We cannot afford to take a step back in the fight against hepatitis B,” he added. “Vaccination is a proven strategy to protect our youngest and most vulnerable citizens.”
The ACIP’s vote is part of ongoing discussions about vaccine administration and public health strategies. The CDC is expected to review the feedback from various stakeholders, including the AMA, before making any final decisions regarding the updated recommendations.
Supporters of maintaining the current guidelines argue that the birth-dose of the hepatitis B vaccine is essential in ensuring high vaccination rates and preventing outbreaks. The AMA is advocating for a more cautious approach, emphasizing the need for comprehensive immunization as a critical component of public health policy.
As this debate unfolds, the AMA plans to continue collaborating with the CDC and other health organizations to advocate for policies that prioritize patient safety and effective disease prevention strategies. The outcome of this situation may influence vaccination practices and public health initiatives for years to come, affecting not only the United States but also setting precedents for vaccination policies in other countries.
In light of this controversy, the AMA urges healthcare professionals, policymakers, and the public to prioritize preventive measures and safeguard the health of vulnerable populations against hepatitis B. The organization remains committed to ensuring that evidence-based practices are upheld in all vaccination recommendations.