A recent study has revealed significant insights into how the bacteria Acinetobacter baumannii develops resistance to antibiotics, presenting challenges for healthcare facilities across the United States. The research highlights the dynamic genomic characteristics of this pathogen, which is responsible for an estimated 1 in 100 hospital-acquired infections.
This study, conducted by scientists at the National Institutes of Health, employed experimental evolution techniques to track genetic changes in A. baumannii. The findings contribute to a growing understanding of the biological mechanisms that enable this bacterium to adapt rapidly to antibiotic treatments.
Understanding the Threat of A. baumannii
A. baumannii is particularly notorious in hospital settings, where it can cause various infections, including pneumonia, bloodstream infections, and wound infections. The bacteria’s ability to acquire antibiotic resistance genes from its environment and other bacteria complicates treatment options.
According to the Centers for Disease Control and Prevention (CDC), A. baumannii is a key target in the fight against antimicrobial resistance, which poses a significant threat to public health. The results of this study underline the need for continued research and surveillance to combat the rise of resistant strains.
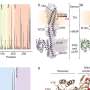
Research Methodology and Findings
The research team implemented a controlled experimental evolution approach, exposing multiple strains of A. baumannii to increasing concentrations of commonly used antibiotics. The study tracked genomic changes over time, revealing specific mutations that conferred resistance.
These mutations affect various biological pathways, including those responsible for cell membrane integrity and antibiotic uptake. The study’s authors emphasized that understanding these mechanisms is crucial for developing new therapeutic strategies to combat A. baumannii infections.
The findings, published in early 2023, not only shed light on the genomic adaptability of A. baumannii but also raise concerns about the potential for similar resistance mechanisms to emerge in other bacterial species. With antibiotic resistance on the rise globally, the implications of this research are far-reaching.
In conclusion, the ongoing battle against drug-resistant bacteria like A. baumannii necessitates innovative research and increased awareness. The insights gained from this study could pave the way for novel treatments and strategies to mitigate the threat of antibiotic-resistant infections in healthcare settings.