URGENT UPDATE: A groundbreaking study reveals a significant advancement in sustainable energy production through electrochemical systems. Researchers from Jiangsu University, the Chinese Academy of Sciences, Hasselt University, and MIT have published a comprehensive review that could revolutionize how we generate energy and chemicals. The findings, published online in eScience in July 2025, address the critical inefficiencies of current electrolysis methods powered by renewable energy.
The reliance on fossil fuels has historically contributed to over 80% of global energy consumption, leading to soaring carbon emissions and significant climate change impacts. The new review highlights the urgent need for integrated electrosynthesis systems to replace the traditional oxygen evolution reaction (OER) with more efficient, value-added reactions. This shift promises to enhance system efficiency while generating valuable by-products.
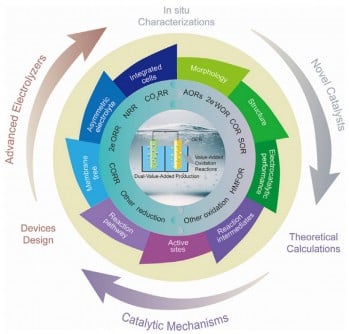
The research team identifies alternative oxidation reactions, such as methanol and glycerol oxidation, as key strategies to replace the sluggish OER. By coupling these reactions with reduction processes, including CO2 reduction and nitrogen reduction, they aim to achieve dual outputs—clean fuels and market-relevant chemicals—while reducing energy consumption. This dual-value approach could ultimately drive a paradigm shift in the chemistry industry, offering solutions to both energy insecurity and environmental degradation.
Researchers emphasize the importance of catalyst development in advancing these systems. Innovations in nanostructured materials, including alloyed and doped catalysts, have expanded active sites and significantly improved selectivity. The use of self-supported and gas-diffusion electrodes is enhancing stability and conversion rates, pushing the boundaries of what can be achieved in industrial-scale applications.
The review discusses the evolution of hybrid electrolyzers, moving from H-type cells to more efficient flow cells and membrane electrode assemblies. These advancements enable the production of green hydrogen and other essential chemicals, addressing pressing climate and resource challenges. Prof. Zhenhai Wen, one of the study’s co-authors, states,
“Electrochemical systems that simultaneously produce two valuable outputs represent a paradigm shift for green chemistry.”
The implications of this research extend far beyond academic interest. By lowering the energy barriers associated with traditional methods and generating high-value chemicals alongside clean fuels, this approach opens new avenues for a sustainable and circular chemical industry. It could also play a crucial role in achieving global net-zero ambitions, creating economic opportunities in renewable-driven industrial chemistry.
As the world grapples with climate change and energy security, the integration of advanced catalysts, novel electrolyzer architectures, and cutting-edge characterization techniques is more urgent than ever. The authors provide a roadmap for future research, emphasizing that the combination of these strategies is essential for transforming chemical manufacturing into a low-carbon, energy-efficient process.
With this significant development, the researchers are setting the stage for a more sustainable future. The study not only highlights the progress made in electrochemical systems but also addresses the challenges that remain. As the world turns its focus to renewable energy sources, the insights from this review could prove vital in shaping the future of energy and chemical production.
Stay tuned for more updates as this research continues to unfold and as industries react to these pioneering advancements. The potential for change is immense, and the time for action is now.