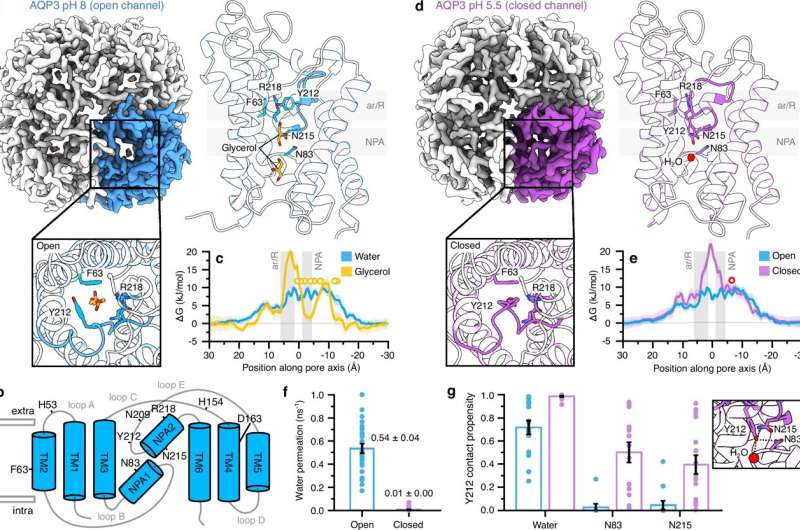
For the first time, researchers at Lund University have demonstrated how cells prevent damage from free radicals, particularly hydrogen peroxide, a molecule that can be both beneficial and harmful. This groundbreaking study, published in Nature Communications, reveals a novel mechanism that allows cells to manage the balance of oxygen molecules essential for their survival.
Cells continuously produce free radicals when they metabolize oxygen. Among these, hydrogen peroxide is crucial for signaling processes in small quantities. However, excessive levels can lead to cellular damage or even death. According to Karin Lindkvist, a professor at Lund University and the study’s lead researcher, previous assumptions suggested that hydrogen peroxide could freely enter cells through membrane channels. The findings of this study challenge that notion, providing new insights into cellular protection mechanisms.
Utilizing advanced cryo-electron microscopy, the research team observed that the channels in the cell membrane remain open under normal conditions, permitting molecules like hydrogen peroxide, water, and glycerol to enter. However, when external hydrogen peroxide concentrations rise beyond a safe threshold, these molecules can accumulate at the channel’s entrance, effectively locking it and preventing further influx.
Lindkvist expressed her astonishment at the discovery, stating, “We were surprised by what we saw. It was like witnessing, in the moment, the cell closing the channel on something that could otherwise cause it harm.” This protective response acts as an automatic safeguard against dangerous levels of hydrogen peroxide entering the cell.
Understanding how cells regulate exposure to free radicals is crucial for advancing medical research, particularly in conditions like diabetes and cancer, where cellular stress is prevalent. Lindkvist noted that cancer cells often produce elevated amounts of free radicals during rapid growth yet manage to survive. This raises the possibility that cancer cells may utilize similar channels to expel excess free radicals, thus avoiding self-inflicted harm.
In future research, Lindkvist’s team aims to investigate whether blocking these channels could effectively kill cancer cells by inhibiting their ability to manage free radicals. This line of inquiry could open new avenues for treatments targeting cancer cell resilience.
The study not only deepens our understanding of cellular defense mechanisms but also highlights the potential for developing novel therapeutic strategies. As research progresses, these findings may lead to significant advancements in treating various diseases linked to cellular stress and oxidative damage.
For more detailed insights, refer to the original research: Peng Huang et al., Structural insights into AQP3 channel closure upon pH and redox changes reveal an autoregulatory molecular mechanism, Nature Communications (2025). DOI: 10.1038/s41467-025-67144-2.